Fluconazole USP EP
Fluconazole USP EP Specification
- EINECS No
- 627-032-6
- Color
- White to off-white
- Structural Formula
- CHCHNCFCHCH(OH)
- Heavy Metal (%)
- Not more than 20 ppm
- Particle Size
- 90% passing through 30 m
- Taste
- Bitter
- HS Code
- 2933.99.80
- Melting Point
- 138-140C
- Poisonous
- Non-poisonous when used as directed
- Solubility
- Soluble in methanol; slightly soluble in water
- Boiling point
- Not applicable (decomposes)
- Storage
- Store in a cool dry place away from direct sunlight
- Ph Level
- 5.5-7.5 (in solution)
- Molecular Weight
- 306.27 g/mol
- Smell
- Odorless
- Shelf Life
- 2 years from the date of manufacture
- Molecular Formula
- C13H12F2N6O
- Loss on Drying
- Not more than 0.5%
- Medicine Name
- Fluconazole USP EP
- Chemical Name
- Fluconazole
- CAS No
- 86386-73-4
- Type
- Antifungal Agent
- Grade
- Pharmaceutical Grade
- Usage
- Treatment of fungal infections
- Purity(%)
- 99%
- Appearance
- White to off-white crystalline powder
- Physical Form
- Solid
About Fluconazole USP EP
Owing to our rich industry experience, we have been able to offer our valuable clients supreme quality Fluconazole USP/EP. The offered chemical is used for the treatment and prophylaxis of fungal infections. Very slightly soluble in water and alcohol, this chemical appears as a white crystalline powder. Processed using the utmost quality basic material and latest production procedures at par with the set industry standards, this Fluconazole USP/EP is offered at industry leading price to the clients.
Features:
- Precise pH value
- Long shelf life
- High effectiveness
- Accurate composition
Product details
|
Chemical Name |
a.-(2,4-Difluorophenyl)-a-(1H-1,2,4,-triazol-1-ylmethyl)-1H-1,2 ,4-triazole-1-ethanol |
|
Molecular Formulae |
C13H12F2N6O |
|
Mol. Wt. |
306.27 |
|
CAS No |
86386-73-4 |
|
Therapeutic Category |
Antifungal |
Pharmaceutical Grade Assurance
Fluconazole USP EP is manufactured under strict quality controls, ensuring a purity level of 99% and compliance with international standards for heavy metals, pH, and appearance. Supplied as a fine crystalline powder, it offers consistency and reliability for pharmaceutical formulations, meeting the requirements set by both the USP and EP.
Key Applications and Usage
Primarily used for the treatment of fungal infections, Fluconazole USP EP acts efficiently against a wide spectrum of fungi. Its high solubility in methanol and stability under recommended storage conditions make it ideal for use by manufacturers, exporters, and suppliers in the pharmaceutical industry.
Safe Storage and Handling
To maintain its efficacy, Fluconazole USP EP should be stored in a cool, dry place away from direct sunlight. It is non-poisonous when used as directed, and its shelf life extends to two years from the date of manufacture, providing a dependable solution for long-term inventory.
FAQs of Fluconazole USP EP:
Q: How should Fluconazole USP EP be stored to maintain its quality?
A: Fluconazole USP EP should be stored in a cool, dry place, protected from direct sunlight. Proper storage conditions help preserve its purity and effectiveness throughout its two-year shelf life.Q: What is Fluconazole USP EP used for in the pharmaceutical industry?
A: Fluconazole USP EP is mainly used as an antifungal agent for the treatment of various fungal infections. Its pharmaceutical grade ensures reliability for use in medicinal formulations manufactured globally.Q: When should Fluconazole USP EP be utilized in a formulation?
A: Fluconazole USP EP should be used as directed in pharmaceutical formulations intended to treat fungal infections. Its use must comply with applicable pharmacopeial standards and directions for safe and effective results.Q: Where does the manufacturing of Fluconazole USP EP typically take place?
A: This product is manufactured, exported, and supplied from India by reputable pharmaceutical companies following USP and EP guidelines to ensure global quality compliance.Q: What is the process for ensuring the purity of Fluconazole USP EP?
A: Fluconazole USP EP undergoes rigorous quality control processes, including testing for purity, heavy metals (no more than 20 ppm), loss on drying, and particle size, ensuring each batch meets pharmaceutical standards.Q: What are the benefits of using Fluconazole USP EP?
A: The primary benefits include its high purity (99%), proven antifungal efficacy, stability in storage, and compliance with recognized quality standards, making it a preferred choice in pharmaceutical manufacturing.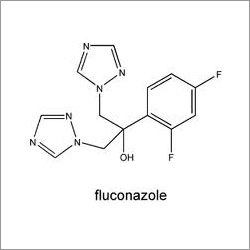

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Active Pharmaceutical Ingredients Category
Beclomethasone Dipropionate USP
Grade : Other, Pharmaceutical Grade
Medicine Name : Beclomethasone Dipropionate USP
Purity(%) : 99%
Chemical Name : Beclomethasone 1721Dipropionate
CAS No : 5534098
Shelf Life : 2 years from manufacturing date if stored properly
Loratadine USP
Grade : Pharmaceutical Grade, Other
Medicine Name : Loratadine USP
Purity(%) : 99%
Chemical Name : Ethyl 4(8chloro56dihydro11Hbenzo(56)cyclohepta(12b)pyridin11ylidene)1piperidinecarboxylate
CAS No : 79794755
Shelf Life : 2 years
Phosphate Disodium Salt
Grade : Industrial Grade
Medicine Name : Phosphate Disodium Salt
Purity(%) : 99%
Chemical Name : Phosphate Disodium Salt
CAS No : 2392394;55203242
Shelf Life : 12 Months


 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS