Loratadine USP
Loratadine USP Specification
- Poisonous
- NO
- Shelf Life
- 2 years
- Melting Point
- 133-136C
- Storage
- Store in a cool dry place away from light and moisture.
- HS Code
- 29333990
- Molecular Weight
- 382.89 g/mol
- Molecular Formula
- C22H23ClN2O2
- Particle Size
- Micronized
- Solubility
- Freely soluble in methanol; practically insoluble in water.
- Heavy Metal (%)
- Not more than 0.002%
- EINECS No
- None
- Structural Formula
- C22H23ClN2O2 (structure not represented here in graphical format)
- Taste
- Bitter
- Loss on Drying
- Not more than 0.5%
- Smell
- Odorless
- Color
- White
- Medicine Name
- Loratadine USP
- Chemical Name
- Ethyl 4-(8-chloro-56-dihydro-11H-benzo(56)cyclohepta(12-b)pyridin-11-ylidene)-1-piperidinecarboxylate
- CAS No
- 79794-75-5
- Type
- Antihistamine
- Grade
- Pharmaceutical Grade
- Usage
- Used to relieve allergy symptoms such as runny nose sneezing and itchy eyes.
- Purity(%)
- 99%
- Appearance
- White to off-white crystalline powder
- Physical Form
- Solid
About Loratadine USP
By keeping track with market development, we are involved in offering our clients Loratadine USP. Used to treat allergies, this drug is closely related to tricyclic antidepressants, such as imipramine. Further, this drug is processed using excellent quality medical grade substances in the most hygiene environment in compliance with the set medical industry standards. This Loratadine USP is also tested on several quality parameters under the supervision of quality experts to ensure its effectiveness.
Features:
- Fast relief
- High effectiveness
- Precise composition
- Purity
Product details
|
Chemical Name |
ibandronate sodium; [1-Hydroxy-3-(methylpentylamino)-propylidene]bisphosphonic acid sodium salt |
|
Molecular Formulae |
C9H22O7NP2Na |
|
Mol. Wt. |
341.21 |
|
CAS No |
138926-19-9 |
Superior Antihistamine for Allergy Relief
Loratadine USP belongs to the class of antihistamines, effectively minimizing allergy symptoms by blocking histamine receptors. Its pharmaceutical-grade purity ensures consistent performance and minimal side effects, making it a preferred choice for managing seasonal allergies.
High Purity and Safe Use
Containing 99% pure active material, Loratadine USP guarantees safety and reliability. The product is not poisonous, non-toxic, and has heavy metal content restricted below 0.002%, ensuring it meets international quality standards for pharmaceutical applications.
Optimal Storage and Handling
To maintain efficacy, Loratadine USP should be stored in a cool, dry location, away from light and moisture. The compound has a shelf life of two years, and its stability is supported by its physical form as a micronized, solid powder.
FAQs of Loratadine USP:
Q: How should Loratadine USP be stored to maintain its shelf life?
A: Loratadine USP should be stored in a cool, dry place, away from direct light and moisture to preserve its efficacy and maintain its two-year shelf life.Q: What benefits does Loratadine USP provide for allergy sufferers?
A: Loratadine USP effectively relieves symptoms like sneezing, runny nose, and itchy eyes by blocking histamine, offering quick and sustained allergy relief with minimal drowsiness.Q: When is Loratadine USP typically used in pharmaceutical applications?
A: It is commonly used in the formulation of allergy medications for both prescription and over-the-counter products, owing to its reliable antihistaminic properties and high purity.Q: Where can Loratadine USP be found or sourced from?
A: Loratadine USP is available through exporters, manufacturers, suppliers, and traders in India, complying with international pharmaceutical standards.Q: What is the production process behind Loratadine USP?
A: It is synthesized through controlled chemical reactions resulting in a micronized, bitter-tasting, white to off-white crystalline powder, ensuring accurate particle sizing and purity.Q: How is Loratadine USP administered to achieve allergy symptom relief?
A: Loratadine USP is processed into oral tablets or other suitable dosage forms for consumption, delivering targeted antihistamine action when taken as directed by healthcare professionals.Q: What makes Loratadine USP suitable for pharmaceutical use?
A: Its high purity (99%), non-toxic nature, precise particle size, and compliance with pharmaceutical-grade standards ensure its efficacy and safety for medicinal formulations.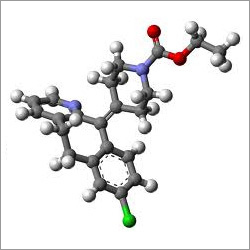

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Active Pharmaceutical Ingredients Category
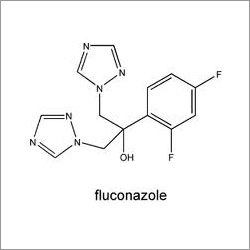
Fluconazole USP EP
CAS No : 86386734
Purity(%) : 99%
Storage : Other, Store in a cool dry place away from direct sunlight
Chemical Name : Fluconazole
Shelf Life : 2 years from the date of manufacture
Usage : Treatment of fungal infections
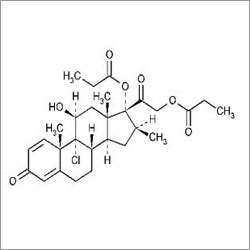
Beclomethasone Dipropionate USP
CAS No : 5534098
Purity(%) : 99%
Storage : Other, Store in a cool dry place; keep container tightly closed.
Chemical Name : Beclomethasone 1721Dipropionate
Shelf Life : 2 years from manufacturing date if stored properly
Usage : Treats asthma allergic rhinitis and other inflammatory conditions.
Phosphate Disodium Salt
CAS No : 2392394;55203242
Purity(%) : 99%
Storage : Room Temperature
Chemical Name : Phosphate Disodium Salt
Shelf Life : 12 Months
Usage : Industrial


 Send Inquiry
Send Inquiry


 Send Inquiry
Send Inquiry Send SMS
Send SMS